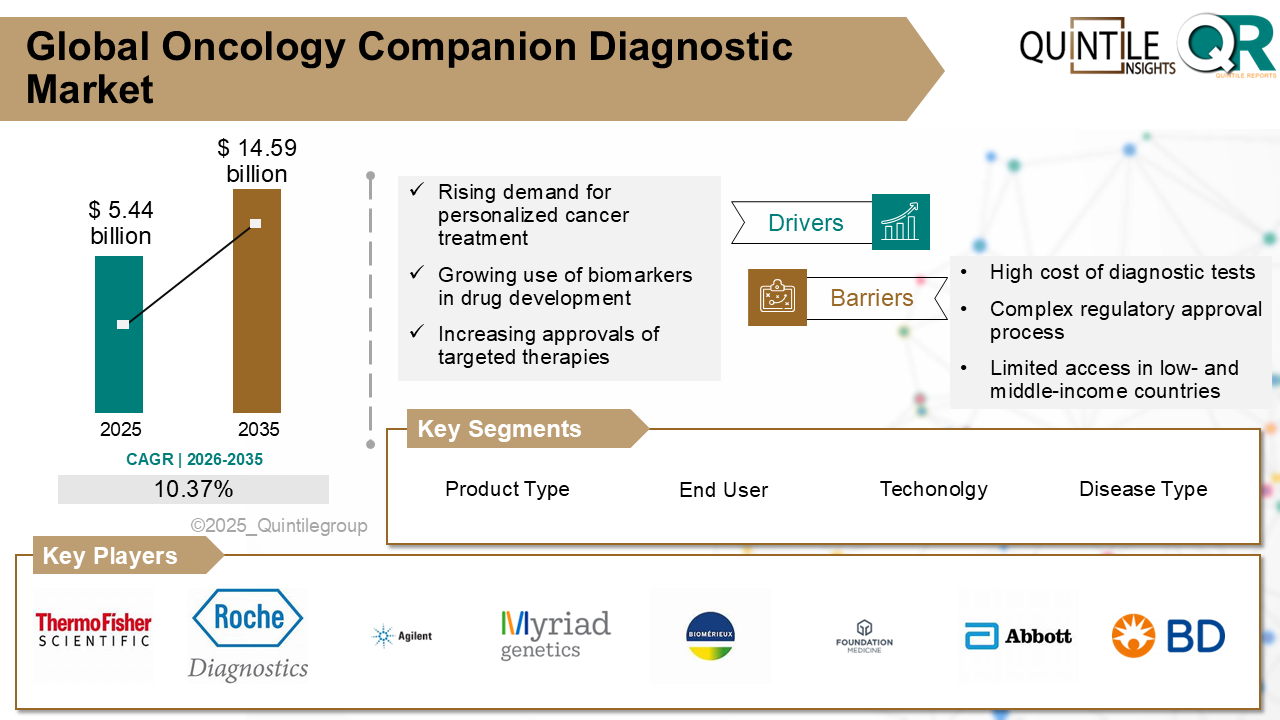
The Global Oncology Companion Diagnostic Market was estimated at USD 5.44 billion in 2026 and is projected to reach USD 14.59 billion by 2035, reflecting a robust CAGR of 10.37% over the forecast period from 2026 to 2035. The Oncology Companion Diagnostic market report offers a comprehensive and nuanced view of the industry, moving beyond conventional analysis. It provides a thorough examination of the markets dynamics, encompassing a detailed exploration of the factors propelling growth, such as evolving economic conditions, advancements in technology, shifts in regulatory policies, and changes in consumer behavior. Furthermore, the report discusses the projected Compound Annual Growth Rate (CAGR), providing stakeholders with a clear understanding of the market's expected growth trajectory and offering data-driven insights into future market dynamics.
The Oncology Companion Diagnostic market under analysis is characterized by dynamic growth and evolving trends that are reshaping the competitive landscape. With 2025 serving as the base year for this Oncology Companion Diagnostic market study, recent data highlights a significant expansion driven by technological advancements, rising consumer demand, and a growing focus on innovation. Companies are refining their go-to-market (GTM) strategies to effectively capture these emerging opportunities and respond to the rapidly changing market dynamics.
Key trends influencing the Oncology Companion Diagnostic market include the rapid adoption of digital technologies, the integration of sustainable practices, and the increasing importance of customer experience. These trends are not only driving growth but also creating new challenges for industry participants, who must adapt their GTM strategies to navigate regulatory changes, supply chain disruptions, and fluctuating economic conditions. Despite these challenges, the Oncology Companion Diagnostic market is poised for sustained growth, with emerging markets playing a critical role in the expansion of the industry.
Looking ahead, the Oncology Companion Diagnostic market is forecasted to continue its upward momentum through 2035, supported by ongoing investments in research and development, strategic partnerships, and mergers and acquisitions. Companies that can effectively tailor their GTM strategies to the evolving market landscape, innovate, and meet shifting consumer demands are likely to achieve sustained success. Oncology Companion Diagnostic market report provides a comprehensive analysis of the current market environment and offers valuable insights into the key drivers, challenges, and opportunities that will shape the industry's future over the next decade. This report offers a comprehensive analysis of market dynamics across various segments, regions, and countries, incorporating both qualitative and quantitative data. It covers the period from 2017 to 2035, providing a detailed examination of historical performance, current market conditions, and future projections.
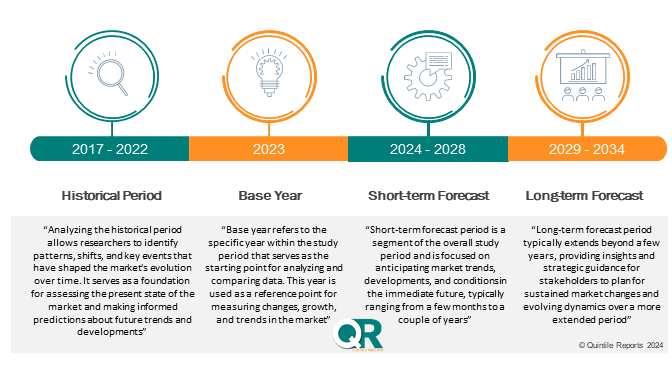
Historical Analysis (2017-2024): The report presents a thorough review of market trends, performance metrics, and growth trajectories for the years 2017 through 2024. This historical perspective is crucial for understanding past market behavior and identifying patterns that influence current and future market dynamics.
Forecast and Projections (2026-2035) : Building on historical data, the report provides forward-looking insights, including market forecasts and growth projections from 2026 to 2035. It details anticipated market trends, emerging opportunities, and potential challenges across different segments, regions, and countries.
Compound Annual Growth Rate (CAGR): The report includes a precise calculation of the compound annual growth rate (CAGR) for the forecast period of 2026 to 2035. This metric will be instrumental in assessing the expected growth trajectory and the overall market potential during the forecast period.
Oncology Companion Diagnostic Market
The Oncology Companion Diagnostic Market plays a critical role in the advancement of precision medicine by offering medical devices and in vitro diagnostics (IVDs) that guide the safe and effective use of cancer therapies. These diagnostics help identify which patients are most likely to benefit from a particular treatment, those at higher risk of adverse reactions, and those who need monitoring during therapy.
Technologies such as Polymerase Chain Reaction (PCR), Next-Generation Sequencing (NGS), Immunohistochemistry (IHC), and In Situ Hybridization (ISH), including Fluorescence in Situ Hybridization (FISH), are commonly employed to support tumour profiling, biomarker identification, and predictive diagnostics. By tailoring treatments to individual patients based on their genetic and molecular profiles, companion diagnostics significantly enhance therapeutic outcomes, minimize harmful side effects, and improve the overall effectiveness of targeted therapies, immunotherapies, and chemotherapies.
North America continues to lead, driven by strong funding from institutions like the National Cancer Institute and a supportive regulatory environment. The U.S. FDAs proactive approvals, such as Illuminas TruSight Oncology Comprehensive test, bolster adoption. Despite challenges like high costs and reimbursement complexities, mature healthcare infrastructure and high cancer incidence support steady market growth.
Europe maintains a leading role, supported by advanced healthcare infrastructure, stringent regulatory frameworks (CE marking), and a growing emphasis on personalized medicine. Germany, France, and the UK are innovation hubs, with collaborations like AstraZenecas partnership with Foundation Medicine expanding access to companion diagnostics. Regulatory harmonization remains a challenge but offers future streamlining potential.
Asia Pacific is the fastest-growing region due to rising cancer burden, aging populations, and increased access to genomic testing. China, India, Japan, and South Korea lead growth, supported by government initiatives and healthcare reforms. Challenges include uneven infrastructure and diverse regulations, but the outlook remains highly promising.
Latin America experiences steady growth with increased cancer burden and gradual precision medicine adoption. Improving healthcare infrastructure and growing awareness help patient access to targeted therapies. Barriers include limited funding, slower regulatory approvals, and uneven technology access.
Middle East & Africa show promising growth potential fueled by rising cancer incidence and healthcare investments. Low current adoption due to funding, awareness, and facility constraints is gradually improving through government and private sector initiatives. Regulatory and infrastructure challenges still need addressing.
Competition hinges on securing timely regulatory approvals through FDA and EMA pathways, granting first-mover advantages. Technological innovation with NGS, liquid biopsies, multiplex panels, and AI platforms enhances diagnostic precision and speed.
Strategic partnerships with pharmaceutical firms ensure diagnostics co-develop and launch alongside cancer drugs. Reimbursement depends on demonstrating clinical utility and cost-effectiveness. Differentiation relies on accuracy, fast turnaround, protocol integration, and patient conveniencefavoring non-invasive liquid biopsies.
Our team of experienced researchers has meticulously gathered and analyzed data to deliver a thorough examination of market dynamics, competitive landscape, and emerging technologies. With a focus on delivering actionable intelligence, this report aims to empower decision-makers with the information needed to make informed choices and stay ahead of the competition. Whether you are a seasoned industry player or a new entrant, our market research report serves as a strategic tool to navigate the complexities of the market, aiding in successful business planning and growth strategies.
This chapter of our Oncology Companion Diagnostic market report provides an in-depth examination of the factors shaping the industry landscape. This section typically encompasses several key elements to offer a comprehensive understanding of the industry landscape such as market drivers & restraints analysis, market opportunities & trend analysis, market size & growth analysis, competitive analysis, SWOT analysis, business environment tools such as Porter's five forces & PESTEL analysis, Ansoff Matrix analysis, penetration & growth prospect analysis, regulatory framework & reimbursement scenario analysis, impact of macro & micro economic factors analysis such as Covid-19 impact, GDP growth, market inflation, U.S.- China trade war, Russia-Ukraine war impact, and supply chain analysis.
The segment analysis chapter of Oncology Companion Diagnostic market report is a critical section that delves into a detailed examination of the market's various segments. Segmentation involves dividing the market into distinct categories based on certain criteria to better understand and address the diverse needs of consumers. This chapter typically follows the introduction and provides a more granular view of the market, offering valuable insights for businesses and stakeholders. The components of the chapter lude segment definitions to understand the inclusions and exclusions of the study, assumptions, market size estimates and growth trend analysis of each segment, qualitative analysis of the segment, technological advancements, market penetration rate, market adoption rate, market share examination by each segment, segment growth drivers and restraint barriers, consumer behaviour and challenge analysis.
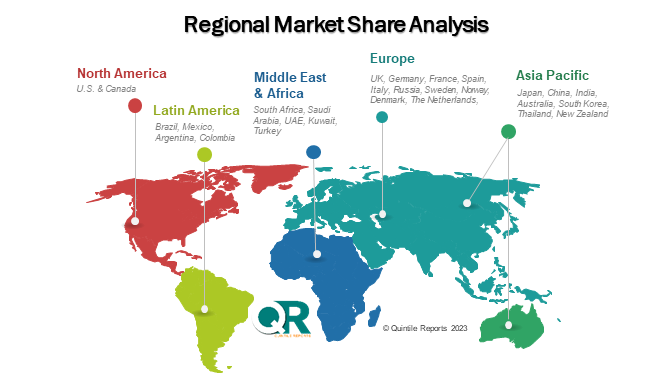
The chapter in Oncology Companion Diagnostic market research report is a pivotal section that examines and predicts the market dynamics and trends specific to different geographical regions. This chapter is crucial for businesses and stakeholders seeking a comprehensive understanding of how the market behaves across various locations, enabling them to tailor strategies and make informed decisions based on regional variations. The regional analysis chapter of our Oncology Companion Diagnostic market report is classified into regions & country-level. The chapter consists of North America (U.S., Canada), Europe (UK, Germany, France, Italy, Spain, Russia, Sweden, Denmark, Norway, Rest of Europe), Asia Pacific (Japan, China, India, Australia, South Korea, Thailand, Rest of Asia Pacific), Latin America (Brazil, Mexico, Argentina, Colombia, Rest of Latin America), Middle East & Africa (South Africa, Saudi Arabia, UAE, Kuwait, Rest of Middle East & Africa).
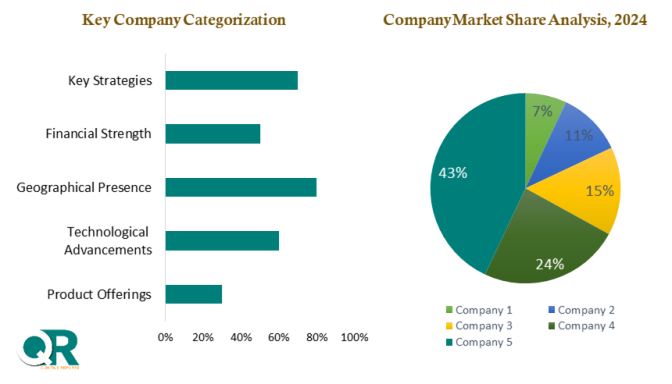
This section of a Oncology Companion Diagnostic market report is a crucial segment that provides a comprehensive overview of the competitive landscape within the market. This section is vital for businesses, investors, and stakeholders seeking insights into key players, their market positioning, strengths, weaknesses, strategies, and potential impacts on the overall market dynamics. The chapter includes research methodology used to analyse the market competition, list of key players operating in the market, detailed company profile section which includes company overview, business verticals, financial performance, product/services benchmarking, geographical presence, and strategic initiatives.
| Report Scope | Details |
| Report Version | 2025 |
| Growth Rate | CAGR of 10.37 from 2026 to 2035 |
| Base year | 2025 |
| Actual estimates/Historical data | 2017 - 2024 |
| Forecast period | 2026 - 2035 |
| Quantitative units | Revenue in USD million/billion & CAGR from 2026 to 2035 |
| Country scope | North America (U.S., Canada), Europe (UK, Germany, France, Italy, Spain, Russia, Sweden, Denmark, Norway, Rest of Europe), Asia Pacific (Japan, China, India, Australia, South Korea, Thailand, Rest of Asia Pacific), Latin America (Brazil, Mexico, Argentina, Colombia, Rest of Latin America), Middle East & Africa (South Africa, Saudi Arabia, UAE, Kuwait, Rest of Middle East & Africa). |
| The Segment covered by Product & Service |
|
| The Segment covered by Allergene Type |
|
| Companies covered |
|
| Report coverage | Revenue forecast, company share, competitive landscape, growth factors, and trends |
| Free customization scope (equivalent to 5 analyst working days) | If you need specific information, which is not currently within the scope of the report, we will provide it to you as a part of the customization |

Statistics for the 2025 Oncology Companion Diagnostic market share, size, and revenue growth rate were created by Quintile Report™. Oncology Companion Diagnostic analysis includes a market forecast outlook for 2035 and a historical overview. Get a free PDF sample of this market analysis, please get in touch with our principal analyst at sales@quintilereports.com
List of Tables
Table 1 List of Abbreviation and acronyms
Table 2 List of Sources
Table 3 North America Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 4 North America Global Oncology Companion Diagnostic Market, by Region, (USD Million) 2017-2035
Table 5 U.S. Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 6 Canada Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 7 Europe Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 8 Europe Global Oncology Companion Diagnostic Market, by Region, (USD Million) 2017-2035
Table 9 Germany Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 10 U.K. Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 11 France Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 12 Italy Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 13 Spain Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 14 Sweden Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 15 Denmark Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 16 Norway Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 17 The Netherlands Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 18 Russia Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 19 Asia Pacific Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 20 Asia Pacific Global Oncology Companion Diagnostic Market, by Region, (USD Million) 2017-2035
Table 21 China Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 22 Japan Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 23 India Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 24 Australia Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 25 South Korea Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 26 Thailand Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 27 Latin America Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 28 Latin America Global Oncology Companion Diagnostic Market, by Region, (USD Million) 2017-2035
Table 29 Brazil Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 30 Mexico Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 31 Argentina Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 32 Middle East and Africa Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 33 Middle East and Africa Global Oncology Companion Diagnostic Market, by Region, (USD Million) 2017-2035
Table 34 South Africa Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 35 Saudi Arabia Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 36 UAE Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 37 Kuwait Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 38 Turkey Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Fig.1 Market research process
Fig.2 Market research approaches
Fig.3 Global Oncology Companion Diagnostic Market: market scenario
Fig.4 Global Oncology Companion Diagnostic Market competitive outlook
Fig.5 Global Oncology Companion Diagnostic Market driver analysis
Fig.6 Global Oncology Companion Diagnostic Market restraint analysis
Fig.7 Global Oncology Companion Diagnostic Market opportunity analysis
Fig.8 Global Oncology Companion Diagnostic Market trends analysis
Fig.9 Global Oncology Companion Diagnostic Market: Segment Analysis (Based on the scope)
Fig.10 Global Oncology Companion Diagnostic Market: regional analysis
Fig.11 Global market shares and leading market players
Fig.12 North America market share and leading players
Fig.13 Europe market share and leading players
Fig.14 Asia Pacific market share and leading players
Fig.15 Latin America market share and leading players
Fig.16 Middle East & Africa market share and leading players
Fig.17 North America, by country
Fig.18 North America
Fig.19 North America market estimates and forecast, 2017-2035
Fig.20 U.S.
Fig.21 Canada
Fig.22 Europe
Fig.23 Europe market estimates and forecast, 2017-2035
Fig.24 U.K.
Fig.25 Germany
Fig.26 France
Fig.27 Italy
Fig.28 Spain
Fig.29 Sweden
Fig.30 Denmark
Fig.31 Norway
Fig.32 The Netherlands
Fig.33 Russia
Fig.34 Asia Pacific
Fig.35 Asia Pacific market estimates and forecast, 2017-2035
Fig.36 China
Fig.37 Japan
Fig.38 India
Fig.39 Australia
Fig.40 South Korea
Fig.41 Thailand
Fig.42 Latin America
Fig.43 Latin America market estimates and forecast, 2017-2035
Fig.44 Brazil
Fig.45 Mexico
Fig.46 Argentina
Fig.47 Colombia
Fig.48 Middle East and Africa
Fig.49 Middle East and Africa market estimates and forecast, 2017-2035
Fig.50 Saudi Arabia
Fig.51 South Africa
Fig.52 UAE
Fig.53 Kuwait
Fig.54 Turkey
The Global Oncology Companion Diagnostic Market was estimated at USD 5.44 billion in 2026 and is projected to reach USD 14.59 billion by 2035, reflecting a robust CAGR of 10.37% over the forecast period from 2026 to 2035. The Oncology Companion Diagnostic market report offers a comprehensive and nuanced view of the industry, moving beyond conventional analysis. It provides a thorough examination of the markets dynamics, encompassing a detailed exploration of the factors propelling growth, such as evolving economic conditions, advancements in technology, shifts in regulatory policies, and changes in consumer behavior. Furthermore, the report discusses the projected Compound Annual Growth Rate (CAGR), providing stakeholders with a clear understanding of the market's expected growth trajectory and offering data-driven insights into future market dynamics.
The Oncology Companion Diagnostic market under analysis is characterized by dynamic growth and evolving trends that are reshaping the competitive landscape. With 2025 serving as the base year for this Oncology Companion Diagnostic market study, recent data highlights a significant expansion driven by technological advancements, rising consumer demand, and a growing focus on innovation. Companies are refining their go-to-market (GTM) strategies to effectively capture these emerging opportunities and respond to the rapidly changing market dynamics.
Key trends influencing the Oncology Companion Diagnostic market include the rapid adoption of digital technologies, the integration of sustainable practices, and the increasing importance of customer experience. These trends are not only driving growth but also creating new challenges for industry participants, who must adapt their GTM strategies to navigate regulatory changes, supply chain disruptions, and fluctuating economic conditions. Despite these challenges, the Oncology Companion Diagnostic market is poised for sustained growth, with emerging markets playing a critical role in the expansion of the industry.
Looking ahead, the Oncology Companion Diagnostic market is forecasted to continue its upward momentum through 2035, supported by ongoing investments in research and development, strategic partnerships, and mergers and acquisitions. Companies that can effectively tailor their GTM strategies to the evolving market landscape, innovate, and meet shifting consumer demands are likely to achieve sustained success. Oncology Companion Diagnostic market report provides a comprehensive analysis of the current market environment and offers valuable insights into the key drivers, challenges, and opportunities that will shape the industry's future over the next decade. This report offers a comprehensive analysis of market dynamics across various segments, regions, and countries, incorporating both qualitative and quantitative data. It covers the period from 2017 to 2035, providing a detailed examination of historical performance, current market conditions, and future projections.
Historical Analysis (2017-2024): The report presents a thorough review of market trends, performance metrics, and growth trajectories for the years 2017 through 2024. This historical perspective is crucial for understanding past market behavior and identifying patterns that influence current and future market dynamics.
Forecast and Projections (2026-2035) : Building on historical data, the report provides forward-looking insights, including market forecasts and growth projections from 2026 to 2035. It details anticipated market trends, emerging opportunities, and potential challenges across different segments, regions, and countries.
Compound Annual Growth Rate (CAGR): The report includes a precise calculation of the compound annual growth rate (CAGR) for the forecast period of 2026 to 2035. This metric will be instrumental in assessing the expected growth trajectory and the overall market potential during the forecast period.

Oncology Companion Diagnostic Market
The Oncology Companion Diagnostic Market plays a critical role in the advancement of precision medicine by offering medical devices and in vitro diagnostics (IVDs) that guide the safe and effective use of cancer therapies. These diagnostics help identify which patients are most likely to benefit from a particular treatment, those at higher risk of adverse reactions, and those who need monitoring during therapy.
Technologies such as Polymerase Chain Reaction (PCR), Next-Generation Sequencing (NGS), Immunohistochemistry (IHC), and In Situ Hybridization (ISH), including Fluorescence in Situ Hybridization (FISH), are commonly employed to support tumour profiling, biomarker identification, and predictive diagnostics. By tailoring treatments to individual patients based on their genetic and molecular profiles, companion diagnostics significantly enhance therapeutic outcomes, minimize harmful side effects, and improve the overall effectiveness of targeted therapies, immunotherapies, and chemotherapies.
North America continues to lead, driven by strong funding from institutions like the National Cancer Institute and a supportive regulatory environment. The U.S. FDAs proactive approvals, such as Illuminas TruSight Oncology Comprehensive test, bolster adoption. Despite challenges like high costs and reimbursement complexities, mature healthcare infrastructure and high cancer incidence support steady market growth.
Europe maintains a leading role, supported by advanced healthcare infrastructure, stringent regulatory frameworks (CE marking), and a growing emphasis on personalized medicine. Germany, France, and the UK are innovation hubs, with collaborations like AstraZenecas partnership with Foundation Medicine expanding access to companion diagnostics. Regulatory harmonization remains a challenge but offers future streamlining potential.
Asia Pacific is the fastest-growing region due to rising cancer burden, aging populations, and increased access to genomic testing. China, India, Japan, and South Korea lead growth, supported by government initiatives and healthcare reforms. Challenges include uneven infrastructure and diverse regulations, but the outlook remains highly promising.
Latin America experiences steady growth with increased cancer burden and gradual precision medicine adoption. Improving healthcare infrastructure and growing awareness help patient access to targeted therapies. Barriers include limited funding, slower regulatory approvals, and uneven technology access.
Middle East & Africa show promising growth potential fueled by rising cancer incidence and healthcare investments. Low current adoption due to funding, awareness, and facility constraints is gradually improving through government and private sector initiatives. Regulatory and infrastructure challenges still need addressing.
Competition hinges on securing timely regulatory approvals through FDA and EMA pathways, granting first-mover advantages. Technological innovation with NGS, liquid biopsies, multiplex panels, and AI platforms enhances diagnostic precision and speed.
Strategic partnerships with pharmaceutical firms ensure diagnostics co-develop and launch alongside cancer drugs. Reimbursement depends on demonstrating clinical utility and cost-effectiveness. Differentiation relies on accuracy, fast turnaround, protocol integration, and patient conveniencefavoring non-invasive liquid biopsies.

Our team of experienced researchers has meticulously gathered and analyzed data to deliver a thorough examination of market dynamics, competitive landscape, and emerging technologies. With a focus on delivering actionable intelligence, this report aims to empower decision-makers with the information needed to make informed choices and stay ahead of the competition. Whether you are a seasoned industry player or a new entrant, our market research report serves as a strategic tool to navigate the complexities of the market, aiding in successful business planning and growth strategies.
This chapter of our Oncology Companion Diagnostic market report provides an in-depth examination of the factors shaping the industry landscape. This section typically encompasses several key elements to offer a comprehensive understanding of the industry landscape such as market drivers & restraints analysis, market opportunities & trend analysis, market size & growth analysis, competitive analysis, SWOT analysis, business environment tools such as Porter's five forces & PESTEL analysis, Ansoff Matrix analysis, penetration & growth prospect analysis, regulatory framework & reimbursement scenario analysis, impact of macro & micro economic factors analysis such as Covid-19 impact, GDP growth, market inflation, U.S.- China trade war, Russia-Ukraine war impact, and supply chain analysis.
The segment analysis chapter of Oncology Companion Diagnostic market report is a critical section that delves into a detailed examination of the market's various segments. Segmentation involves dividing the market into distinct categories based on certain criteria to better understand and address the diverse needs of consumers. This chapter typically follows the introduction and provides a more granular view of the market, offering valuable insights for businesses and stakeholders. The components of the chapter lude segment definitions to understand the inclusions and exclusions of the study, assumptions, market size estimates and growth trend analysis of each segment, qualitative analysis of the segment, technological advancements, market penetration rate, market adoption rate, market share examination by each segment, segment growth drivers and restraint barriers, consumer behaviour and challenge analysis.
The chapter in Oncology Companion Diagnostic market research report is a pivotal section that examines and predicts the market dynamics and trends specific to different geographical regions. This chapter is crucial for businesses and stakeholders seeking a comprehensive understanding of how the market behaves across various locations, enabling them to tailor strategies and make informed decisions based on regional variations. The regional analysis chapter of our Oncology Companion Diagnostic market report is classified into regions & country-level. The chapter consists of North America (U.S., Canada), Europe (UK, Germany, France, Italy, Spain, Russia, Sweden, Denmark, Norway, Rest of Europe), Asia Pacific (Japan, China, India, Australia, South Korea, Thailand, Rest of Asia Pacific), Latin America (Brazil, Mexico, Argentina, Colombia, Rest of Latin America), Middle East & Africa (South Africa, Saudi Arabia, UAE, Kuwait, Rest of Middle East & Africa).

This section of a Oncology Companion Diagnostic market report is a crucial segment that provides a comprehensive overview of the competitive landscape within the market. This section is vital for businesses, investors, and stakeholders seeking insights into key players, their market positioning, strengths, weaknesses, strategies, and potential impacts on the overall market dynamics. The chapter includes research methodology used to analyse the market competition, list of key players operating in the market, detailed company profile section which includes company overview, business verticals, financial performance, product/services benchmarking, geographical presence, and strategic initiatives.

| Report Scope | Details |
| Report Version | 2025 |
| Growth Rate | CAGR of 10.37 from 2026 to 2035 |
| Base year | 2025 |
| Actual estimates/Historical data | 2017 - 2024 |
| Forecast period | 2026 - 2035 |
| Quantitative units | Revenue in USD million/billion & CAGR from 2026 to 2035 |
| Country scope | North America (U.S., Canada), Europe (UK, Germany, France, Italy, Spain, Russia, Sweden, Denmark, Norway, Rest of Europe), Asia Pacific (Japan, China, India, Australia, South Korea, Thailand, Rest of Asia Pacific), Latin America (Brazil, Mexico, Argentina, Colombia, Rest of Latin America), Middle East & Africa (South Africa, Saudi Arabia, UAE, Kuwait, Rest of Middle East & Africa). |
| The Segment covered by Product & Service |
|
| The Segment covered by Allergene Type |
|
| Companies covered |
|
| Report coverage | Revenue forecast, company share, competitive landscape, growth factors, and trends |
| Free customization scope (equivalent to 5 analyst working days) | If you need specific information, which is not currently within the scope of the report, we will provide it to you as a part of the customization |

Statistics for the 2025 Oncology Companion Diagnostic market share, size, and revenue growth rate were created by Quintile Report™. Oncology Companion Diagnostic analysis includes a market forecast outlook for 2035 and a historical overview. Get a free PDF sample of this market analysis, please get in touch with our principal analyst at sales@quintilereports.com
Table 1 List of Abbreviation and acronyms
Table 2 List of Sources
Table 3 North America Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 4 North America Global Oncology Companion Diagnostic Market, by Region, (USD Million) 2017-2035
Table 5 U.S. Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 6 Canada Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 7 Europe Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 8 Europe Global Oncology Companion Diagnostic Market, by Region, (USD Million) 2017-2035
Table 9 Germany Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 10 U.K. Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 11 France Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 12 Italy Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 13 Spain Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 14 Sweden Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 15 Denmark Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 16 Norway Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 17 The Netherlands Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 18 Russia Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 19 Asia Pacific Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 20 Asia Pacific Global Oncology Companion Diagnostic Market, by Region, (USD Million) 2017-2035
Table 21 China Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 22 Japan Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 23 India Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 24 Australia Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 25 South Korea Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 26 Thailand Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 27 Latin America Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 28 Latin America Global Oncology Companion Diagnostic Market, by Region, (USD Million) 2017-2035
Table 29 Brazil Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 30 Mexico Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 31 Argentina Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 32 Middle East and Africa Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 33 Middle East and Africa Global Oncology Companion Diagnostic Market, by Region, (USD Million) 2017-2035
Table 34 South Africa Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 35 Saudi Arabia Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 36 UAE Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 37 Kuwait Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Table 38 Turkey Global Oncology Companion Diagnostic Market, by Segment Analysis, (USD Million) 2017-2035
Fig.1 Market research process
Fig.2 Market research approaches
Fig.3 Global Oncology Companion Diagnostic Market: market scenario
Fig.4 Global Oncology Companion Diagnostic Market competitive outlook
Fig.5 Global Oncology Companion Diagnostic Market driver analysis
Fig.6 Global Oncology Companion Diagnostic Market restraint analysis
Fig.7 Global Oncology Companion Diagnostic Market opportunity analysis
Fig.8 Global Oncology Companion Diagnostic Market trends analysis
Fig.9 Global Oncology Companion Diagnostic Market: Segment Analysis (Based on the scope)
Fig.10 Global Oncology Companion Diagnostic Market: regional analysis
Fig.11 Global market shares and leading market players
Fig.12 North America market share and leading players
Fig.13 Europe market share and leading players
Fig.14 Asia Pacific market share and leading players
Fig.15 Latin America market share and leading players
Fig.16 Middle East & Africa market share and leading players
Fig.17 North America, by country
Fig.18 North America
Fig.19 North America market estimates and forecast, 2017-2035
Fig.20 U.S.
Fig.21 Canada
Fig.22 Europe
Fig.23 Europe market estimates and forecast, 2017-2035
Fig.24 U.K.
Fig.25 Germany
Fig.26 France
Fig.27 Italy
Fig.28 Spain
Fig.29 Sweden
Fig.30 Denmark
Fig.31 Norway
Fig.32 The Netherlands
Fig.33 Russia
Fig.34 Asia Pacific
Fig.35 Asia Pacific market estimates and forecast, 2017-2035
Fig.36 China
Fig.37 Japan
Fig.38 India
Fig.39 Australia
Fig.40 South Korea
Fig.41 Thailand
Fig.42 Latin America
Fig.43 Latin America market estimates and forecast, 2017-2035
Fig.44 Brazil
Fig.45 Mexico
Fig.46 Argentina
Fig.47 Colombia
Fig.48 Middle East and Africa
Fig.49 Middle East and Africa market estimates and forecast, 2017-2035
Fig.50 Saudi Arabia
Fig.51 South Africa
Fig.52 UAE
Fig.53 Kuwait
Fig.54 Turkey

A license granted to one user. Rules or conditions might be applied for e.g. the use of electric files (PDFs) or printings, depending on product.
A license granted to multiple users.
A license granted to a single business site/establishment.
A license granted to all employees within organisation access to the product.
Immediate / Within 24-48 hours - Working days
Online Payments with PayPal and CCavenue
You can order a report by picking any of the payment methods which is bank wire or online payment through any Debit/Credit card or PayPal.
Hard Copy
Minimal Residual Disease Testing Market Report SummaryThe Global Minimal Residual Disease Testing Ma
Read MoreReport Descriptions: The Global Companion Animal Diagnostics Market was estimated at USD 6.95 billio
Read MoreReport Descriptions: The Global Cryoablation Probe Market was estimated at USD 216.09 million in 202
Read More